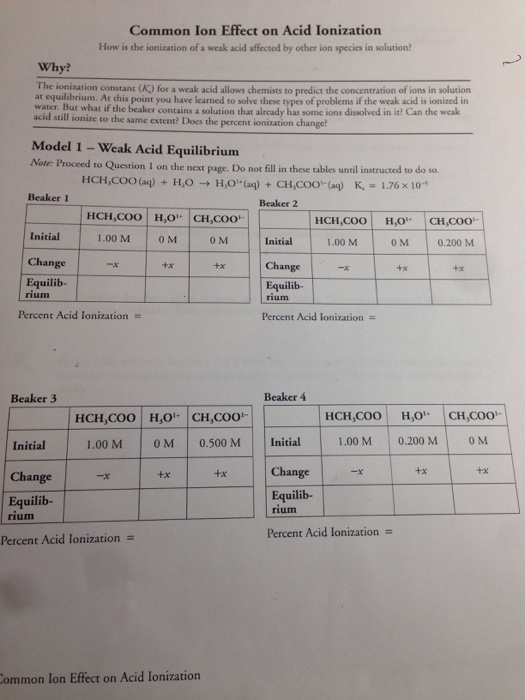
Common Ion Effect on Acid Ionization
The weak acid ionization constant for hydrofluoric acid is 631 x 10- Beaker 1 0025 M HF Beaker 2 0025 M HIF 0010 M NaF Beaker 3 0025 M HF 0010 M Bak Beaker 4 0025 M HIF 0010МН M HCI Common Ion Effect on Acid Ionization 12. After watching this video you will be able to.

Ap Chem Buffers Pogil Buffers How Can A Solution Neutralize Both Acids And Bases Why Buffer Solutions Are A Mixture Of Substances That Have A Fairly Course Hero
Common ion effect is the suppression of ionization of a weak electrolyte by the presence in the same.

. The role that the common ion effect plays in solutions is mostly visible in the decrease of solubility of solids. A common ion is added. Support your answer with evidence from Model 1.
Describe the effect of common ions on the percent ionization of weak acids and bases. Buffering solutions contain either an acid or base accompanied by its conjugate. It is a special case of the LeChatelier principle.
This topic also discuss the effect of a common ion on the dissociation of weak acids in water. The common ion effect also plays a role in the regulation of buffers. The effect suppresses the ionization of a weak acid or base by adding more common ions.
The phenomenon in which the degree of dissociation of any weak electrolyte is suppressed by adding a small amount of strong electrolyte containing a common ion is called a common ion effect. Compare the per- acid in water with its percent ionization in 0500 M. Add your answer and earn points.
A salt that has an ion in common with the weak electrolyte Example Acetic acid CH 3 COOH is a weak acid with the following ionization reaction. The source of the common ion is typically provided by adding a strong acid a strong base or. Some of the salt will be precipitated until the ion product is equal to the solubility product.
Find the pH in a solution of 01 M lactic acid and 01 M sodium lactate Given. This suppresses the ionization of a weak acid or a weak base. Ka Hcommon ion acid Kb OH-common ion base can decrease the solubility of certain slightly soluble salts.
The presence of common ion with a weak acid decreases the percent ionization of. The dissociation of a weak electrolyte is decreased by adding to the solution a strong electrolyte ie. Dissociation of a Weak Acid.
The common ion effect is the phenomenon in which the addition of an ion common to two solutes causes precipitation or reduces ionization. The common ion effect is a way to understand how the different ions affect solubility and ionization of other molecules in a solution. The presence of common ion with a weak acid decreases the percent ionization of the weak acid.
As seen in water the weak acid has a 042 ionization but when a common ion was present the percent ionization changed to 00088 and 00035. Plancks Quantum Theory and Black Body Radiation. An example of the common ion effect is when sodium chloride NaCl is added to a solution of HCl and water.
Common ion effect occurs when a weak acid and its conjugate base or a weak baseconjugate acid are both present in a solution. At chis point you have learned to solve these types of problems if the weak acid is ionized in. Provide two things that could be done to a solution to increase the percent ionization of a weak acid.
171 The Common Ion Effect The dissociation of a weak electrolyte is decreased by the addition of a strong electrolyte that has an ion in common with the weak electrolyte. In the case of an acidic buffer the hydrogen ion concentration decreases and the resulting solution is less acidic than a solution that contains the pure weak acid. Common Ion Effect on Acid Ionization 5.
The ionization constant K for a weak acid allows chemists to predict the concentration of ions in solution ar equilibrium. It is considered to be a consequence of Le Chatliers principle or the Equilibrium Law. The common ion effect is an effect that suppresses the ionization of an electrolyte when another electrolyte which contains an ion which is also present in the first electrolyte ie.
Common ion effect It states that if the concentration of any one of the ions is increased then according to Le Chateliers principle some of the ions in excess should be removed from the solution by combining with the oppositely charged ions. You have seen that the presence of a common ion in solution will decrease the percent ionization of a weak acid in solution. The acid ionization constant for benzoic acid CHCOOH İs 646 10-s.
Less acid will dissociate The Common Ion Effect. The shift in equilibrium caused by the addition of a substance having an ion in common with the equilibrium mixture. Addition of the common ion causes the equilibrium to shift left.
Common-ion effect - ionization of electrolyte decreases if common ion added shifts equilibrium against a certain side. Through the addition of common ions the solubility of a compound generally decreases due to a shift in equilibrium. This decreases the reaction quotient because the reaction is being forced towards the left to reach equilibrium.
Determine the pH of the solution made from the weak acid weak base in the presence of the common ion. When a common ion is added to a weak acid or a weak base it prevents the solution from ionizing as it would have done otherwise. For example consider the ionization of a weak acid acetic acid.
HC 2H 3O 2aq H aq C 2H 3O 2 aq If we add additional C 2H 3O 2 ions by the. Now consider the common ion impact of ceOH- ón the ionization óf ammonia. The common ion impact suppresses the iónization of a vulnerable acid by including even more of an ion that is usually a item of this equilibrium.
Find an answer to your question common ion effect on acid ionization pogil clarissaoh522 clarissaoh522 03112021 Chemistry High School answered Common ion effect on acid ionization pogil 1 See answer Advertisement Advertisement clarissaoh522 is waiting for your help. The addition of cyanide ions will reduce hydrogen cyanide ionization and shift the equilibrium of the left and change its balance to the left. The common ion effect suppresses the ionization of a weak acid by adding more of an ion that is a product of this equilibrium.
CH 3 COOH H 2 O H 3 O CH 3 COO-Ka 18 x 10-5. The addition of a common ion or an ion shared between. Common Ion Effect on Acid Ionization How is the ionization of a weak acid affected by other ion species in solution.
When a common ion is added to a weak acidbase is equivalent to adding stress to the system.
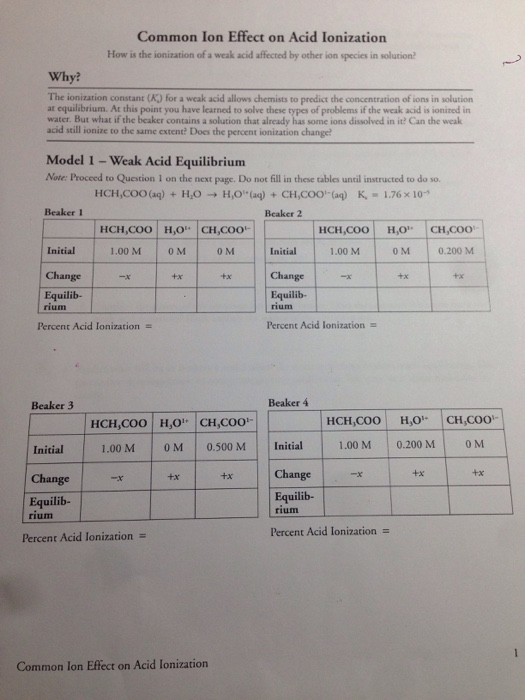
Solved Common Ion Effect On Acid Ionization How Is The Chegg Com
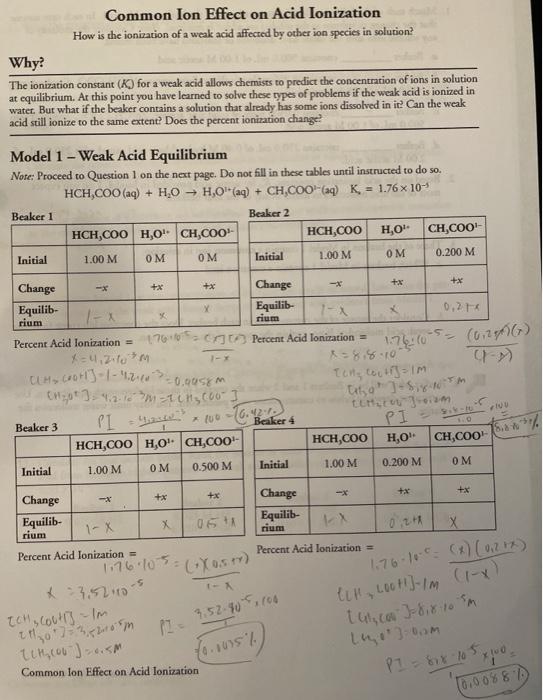
Solved Common Ion Effect On Acid Ionization How Is The Chegg Com

Common Ion Effect And Buffers Chemistry Khan Academy Youtube
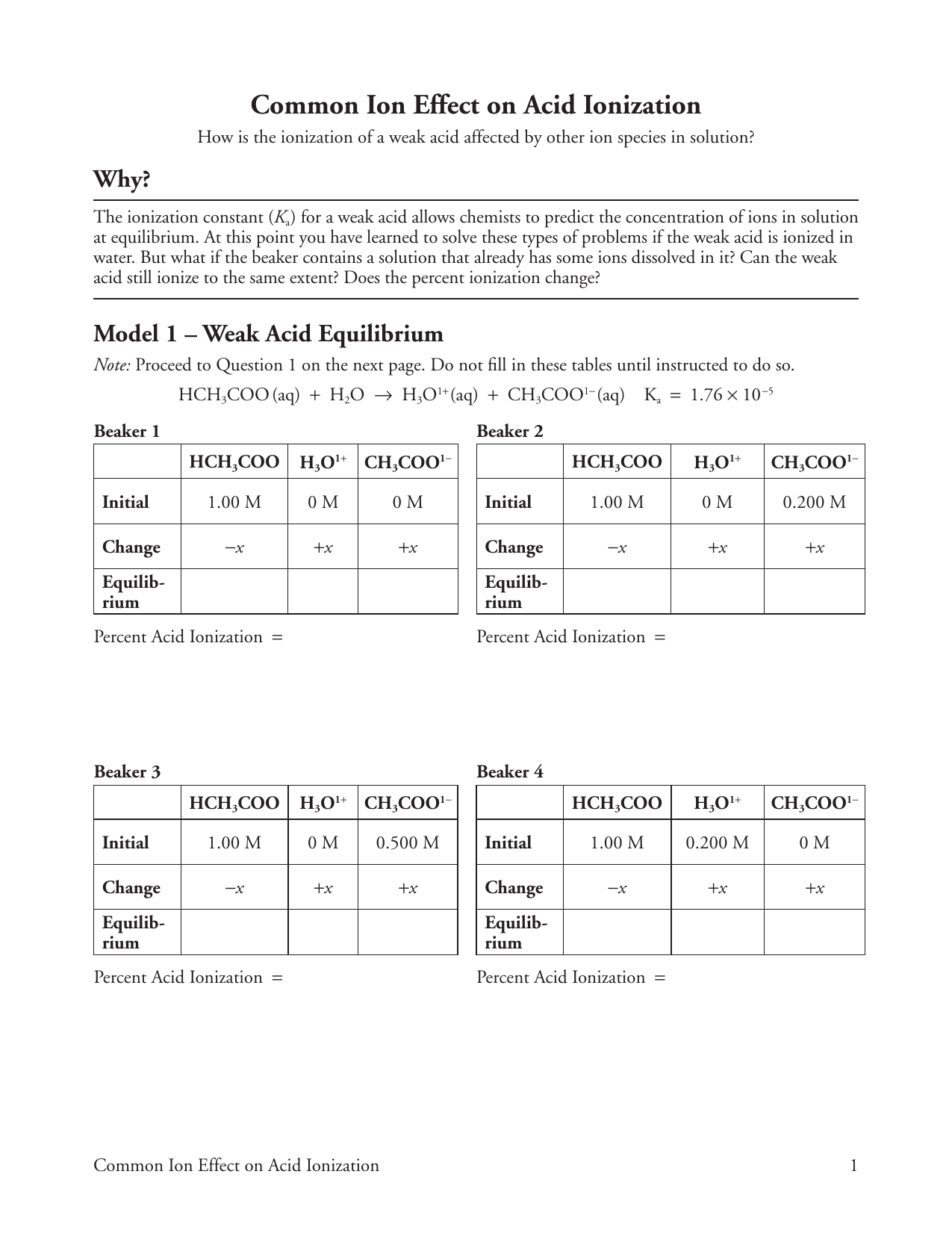
24 Common Ion Effect On Acid Ionization

Common Ion Effect Shift In Equilibrium Position That Occurs Because Of The Addition Of An Ion Already Involved In The Equilibrium Reaction An Application Ppt Download

Chapter 17 Acid Base Solubility Equilibria Ppt Download

Common Ion Pogil Pdf Common Ion Effect On Solubility How Is The Solubility Of A Solid Affected By Other Ion Species In Solution Why The Solubility Course Hero

Solved Common Ion Effect On Acid Ionization How Is The Chegg Com

24 Common Ion Effect On Acid Ionization Answers Model Note Proceed To Questi Hch3coo Aq H20 9 H3 Percen Comm Onization Of A Weak How Is The Course Hero
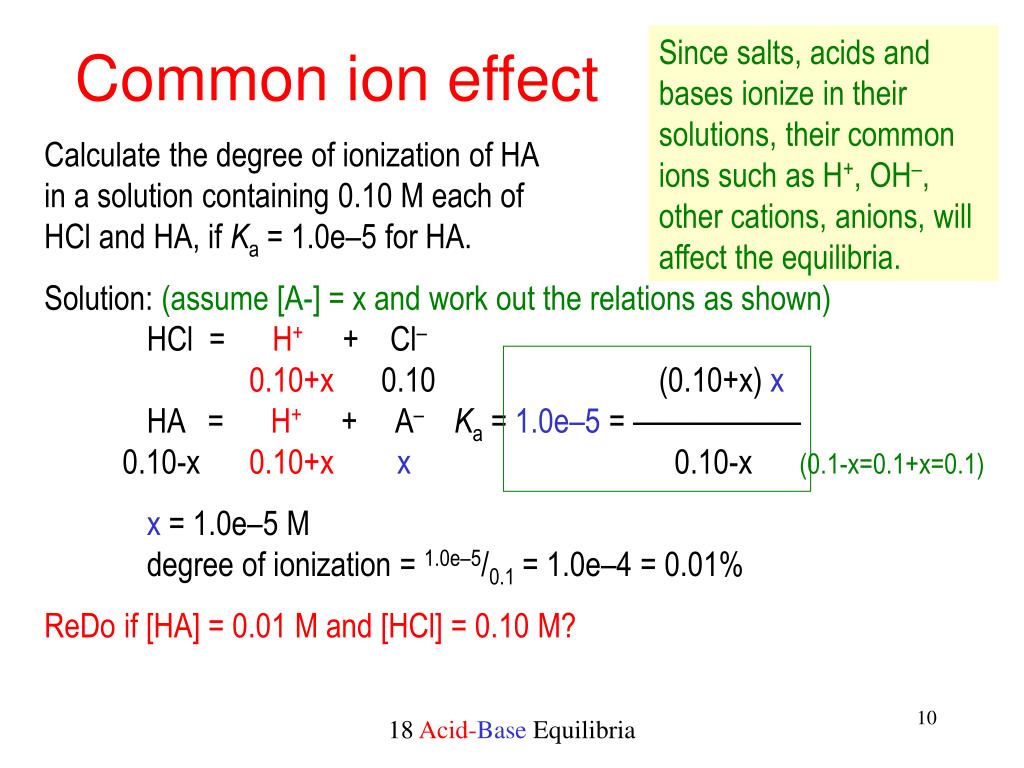
Ppt 18 Additional Aspects Of Acid Base Equilibria Powerpoint Presentation Id 550560

Chapter 17 Acid Base Equilibria Part I 1dr Al Saadi Ppt Download
Solved Common Ion Effect On Acid Ionization How Is The Chegg Com


Comments
Post a Comment